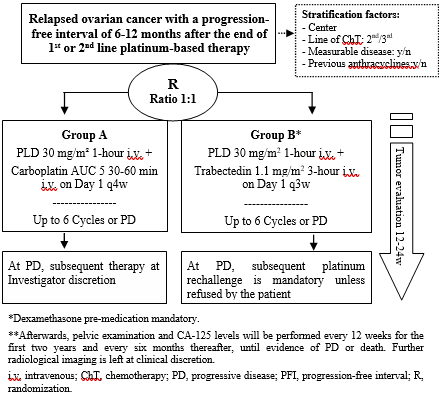
Dr. Mansoor Raza Mirza speaking at The Global Academy of Women’s Cancer in Madrid, Spain 2023
Privacy Policy for NSGO
NSGO treats personal information and complies with the laws and principles of good data processing practices. Therefore, we have adopted this Privacy Policy, which briefly describes how we treat personal information, so legal and transparent processing is ensured.
We are responsible for processing your personal information:
NSGO is the data controller and you can always contact the organization’s data protection officer:
Mansoor Raza Mirza
Nordic Society of Gynecologic Oncology – Clinical Trial Unit
Copenhagen University Hospital
Department of Oncology, 9431
Blegdamsvej 60
DK-2100 Copenhagen
Office phone: +45 35 45 33 78
fax: +45 3545 2898
Mansoor.Raza.Mirza@regionh.dk.
We treat the following personal information about members:
- General personal information: For example, contact information such as name, work address, work phone number, private phone number, date of birth, email address, country, work title, date of membership, date of membership termination and cv in relation to membership application. Parts of credit card number via Epay and, if applicable, Bank account number for reimbursement purposes. When applicable, Board Members may be asked to give NSGO copies of other personal documents (for example passport or tax bill) in relation to the Danish Laundering Act.
- Other information: For example, positions of trust, board membership and other duties in relation to the organization.
We collect personal information from:
Usually we will get the information from you, when you register to become a member of NSGO and when you apply for reimbursements or pay your membership fee
The organization’s legal basis to process personal information:
- That we as an organization have a (legitimate) interest in processing your information
- That it is necessary for us to fulfill an agreement with you
- You have agreed that we can process your information via becoming a member of the
The purpose of processing personal information:
- That we can run the organization’s activities, including planning, implementing and following up on them
- To invite to vote for Board and President Elect elections
- To distribute newsletters
- To comply with legislation
- To deliver services, in the form of the possibility to participate in the Annual Meeting, Investigator Meeting and if applicable Strategic Meetings, Board Meetings, Scientific Committee Meetings, Foundation Meetings.
- To notify members about other Gynecologic Cancer Managing your relationship with the organization
- To handle your membership rights
- For you to fulfill your obligations as a member of the organization, including paying the membership fee, etc.
- Being able to display situation images from a specific event in the organization for instance from NSGO Annual Meetings
- To maintain historical value data for statistics and the like
Consent:
When you become a member, we have a legal basis to process your personal information for the (legitimate) interest of the organization. However, it may happen that we judge it necessary to obtain your written consent for other purposes. In that case you will have the opportunity to voluntarily agree, and you may withdraw it at any time by notifying us.
Dispatch of your personal information:
We do not disclose information about you to anyone outside the organization without your consent, however, it should be noted, that if you e.g. accept an invitation to a meeting, your personal information might be forwarded to the meeting location or the meeting organizer. Only personal information relevant for the 3rd party will be disclosed in these circumstances.
Storing and deleting your personal information:
For historical reasons and as your details might be saved in old attendance lists, email correspondence and minutes from meetings, we cannot promise that all personal information about you will be deleted, if you terminate your membership.
Your rights:
You have a number of rights via the General Data Protection Regulation (GDPR):
- The Right to Information (to be informed what information we process about you)
- The Right to Rectification (to have false information about you corrected)
- The Right to Restriction of processing (under certain conditions, to have the processing of your data restricted)
- The Right to Object (to object to your information being used for wrong purposes)
You may make use of your rights by contacting us. If you submit a request to have your personal information corrected or deleted, we will investigate whether the conditions are met and, in that case, make changes or deletions as soon as possible and, if applicable, also notify our data processors to do so.
You can file a complaint with the supervisory authorities – The Danish ‘Datatilsynet’ – About processing your personal information (www.datatilsynet.dk).
Revision of Privacy Policy:
We reserve the right to change the organization’s privacy policy. When we change privacy policy, we also change the date and version number at the bottom of this document. In case of significant changes, you will receive a notification.
The current privacy policy is always available on our website.

Practice Areas
Newsletter
Sign up to our newsletter