An overview of disease, diagnosis and treatment
The cervix and cervical canal are the most distal parts of the uterus.
Pre-malignant cervical lesions can be detected on screening smear examinations. A smear tests is a screening tool, and in case of suspicious cervical lesions/tumor, the patient must be referred for specialized conclusive examination to determine, if a lesion is malignant or not.
Patients with severe dysplasia or carcinoma in situ (pre-malignant cervical lesion) may be recommended to undergo conisatio of the cervix, to prevent progression to actual cervical cancer.
Within the last decades extensive screening programs were introduced, especially in developed countries. The number of deaths from cervical cancer has effectively declined with the introduction of the screening programs and also HPV-vaccination.
Cervical cancer differs from uterine (endometrial) cancer regarding symptoms/presentation, histology, treatment and diagnosis.
Epidemiology
The majority of cervical cancers are related to persistent Human Papilloma Virus (HPV) infection, especially with the high-risk oncogenic HPV types 16 & 18.
Patients diagnosed with cervical cancer include both very young patients in their 20’s, as well as older women.
The prognosis is dependent on the tumor stage and cell-type(s) of the cancer. It significantly affects the outcome for the patient, if the tumor has spread to local pelvic lymph nodes, para-aortic lymph nodes, bladder, rectum, or distant organs.
Diagnosis
A suspicion of cervical cancer can be based on results from smear screening, or clinically based on symptoms, or objective findings during a gynaecological examination.
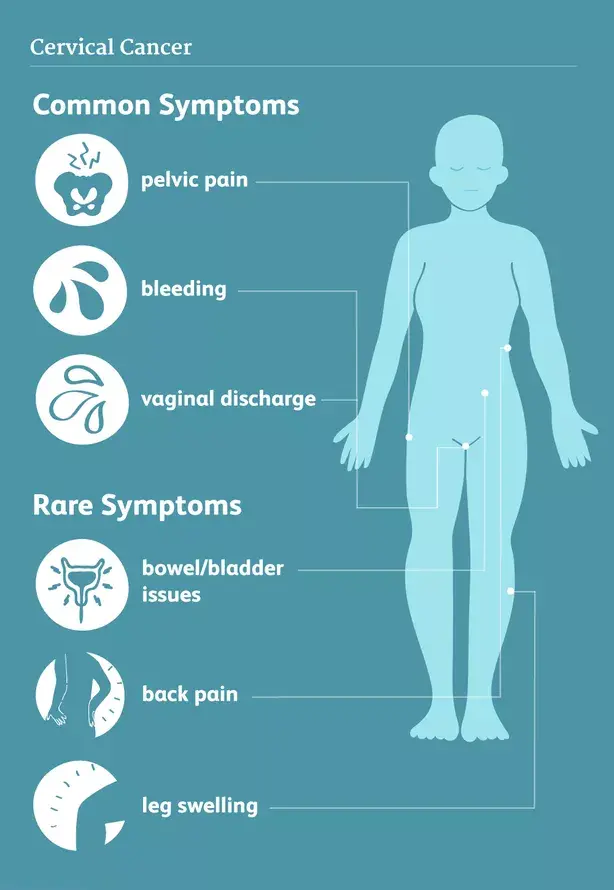
Often early stages of cervical cancer do not give symptoms. Later on patients may experience bleeding, contact-bleeding during intercourse, changes in vaginal discharge, pain, bladder/bowel symptoms or leg-swellling.
Upon a suspicion of cervical malignancy patients must be referred for specialist gynaecological examination, incl. colposcopy (microscopic examination of the cervical surface lining), biopsies, and scraping from the cervical canal. All tissue samples (biopsies) taken out are send for histological verification.
The prognosis of cervical cancer depends on the histological type, stage and possible spread of the cancer at initial diagnosis.
In many specialized facilities treating patients with cervical cancer, the woman (with cervical cancer over stage 1A) is clinically staged under full anesthesia. If involvement of bladder or rectum is suspected, a cystoscopy and/or rectoscopy must also be performed.
The extent of disease and possible spread to lymph nodes/distant organs are evaluated by radiological examination with either PET/CT, CT or MRI scan, before deciding upon the treatment plan.
Examinations
Cervical Colposcopy
Cervical colposcopy is performed by specialist gynaecologists. The colposcope (microscopic tool) is inserted through the vagina during an ordinary gynaecological examination. Through the microscope the doctor can inspect for abnormal lining, tumors, and bleeding of the portio (cervical mouth).
In case of suspicious area(s), or abnormal tissue/cells, the doctor performs biopsies/cervical scraping, and the retrieved tissue samples are send for histological examination.
HPV Test
Infection with Human Papilloma Virus (HPV) can be detected by examining for genetic material from HPV. The majority of cases of HPV infection is cleared by patients spontaneously. However, in a minority with persistent HPV infection, it may lead to pre-cancerous or even cancerous lesion in the cervix. Therefor regular screening smear test is recommended, for possible lesions to be detected early.
Smear Test (Pap Test / Pap Smear)
During a gynaecological examination with smear test, cells are retrieved from the cervical surface lining. The material is send for pathological examination, where the histologically stained cells are inspected through a microsope.
More specifically, cervical cells are removed by scraping and brushing the cervix, then spread on a slide, stained with a special staining solution (which identifies atypical/malignant cells), and then microscopically quantified.
A smear test is used as a screening tool for the early detection of abnormal or cancerous cells, and its preliminary stages. If actual cancer is suspected, then a smear test is insufficient, and the patient must have representative biopsies taken and examined for histological verification.
Treatment options
Surgery
The earliest stage of cervical cancer can be treated with limited surgery (conization), where a cone-shaped piece of the distal cervical tissue (incl. the tumor) is resected. The rest of the cervix is left intact. The operation is performed via the vagina, under local or general anesthesia. After a conization a woman can still become pregnant, as the rest of the cervix and the uterus are left intact.
With more advanced stage and larger cervical cancers, a conization is insufficient. A limited number of patients may be treated with a surgical procedure called a trachelectomy, where a larger part of the cervix is removed. Again, this will make pregnancy an option for the younger patients.
The most advanced form of surgery is a hysterectomy, where the cervix and uterus are removed along with adjacent connective tissue and lymph nodes. The procedure is performed under general anesthesia via the vagina, or through an abdominal incision.
The abovementioned surgical procedures are all curatively intended, if they are performed by skilled surgeons on sufficiently and correct staged patients.
Radiation Therapy
Radiation therapy uses high-powered energy beams (usually fotons) to destroy and kill cancer cells. The cervical tumor and uterus etc. is irradiated from the outside through the skin (external beam radiation therapy), or from the inside through the vagina (brachytherapy). In a range of patients a combination of the two modalities is recommended for sufficient effect.
During radiation treatment care is taken to protect the surrounding normal tissue such as bladder, rectum, bowel, kidneys etc. However, it is often unavoidable that a limited dose affects the surrounding tissue, and consequentially results in side effects of varying degree.
The side effects may be urinary problems, diarrhea, inflammation of the mucous membranes in the vagina, bladder, rectum and bowel, as well as nausea, discomfort/pain, development of scar-tissue in the pelvic area (fibrosis), and swelling af the leg(s) due to lymphoedema. Brachytherapy can result in shortening and hardening (due to fibrosis) of the top of the vagina, resulting in sexual disabilities.
Always discuss the risks and possible treatment options with your radiation oncologist prior to commencing your treatment.
When radiation therapy is combined with chemotherapy as treatment for locally advanced cervical cancers (usually stage 2-3), it is referred to as chemoradiation treatment. It may also be given following surgery for a group of high risk patients to decrease their risk of recurrence of cancer.
Chemotherapy And Antibody Therapy
For recurrent or disseminated cervical cancer, where curative treatment is not an option, chemotherapy and antibody treatment can be given intravenously to decrease tumor burden and alleviate symptoms for the patients. The addition of antibody therapy to combination chemotherapy, have demonstrated good effects, and can hopefully prolong life for treated patients.
The antibody targets cancer cells by blocking their blood supply and thereby inhibiting tumor growth.
The combination treatment can be associated with side effects, and it is important to discuss the risks and possible treatment options with your oncologist prior to starting treatment.
Making Decisions About Your Care
The choice of therapy for your cancer is an individual decision, and many factors are taken in to consideration, when your doctor(s) discuss the best option(s) with you. These include the type, size and location/spread of the tumor, as well as your age and general health status.
We recommend, that you have a thorough discussion with your doctor(s) about the results upon your diagnosis, the treatment-plan and your chances of recovery (e.g. prognosis).
You are encouraged to include family and/or friends for participation in doctors appointments etc. for improving your/their understanding and remembrance of relevant information, as well as for emotional and physical support during appointments and treatments.
Your doctor can give a detailed explanation of the various therapy options, and has knowledge of the possibility to participate in a clinical trial. Each patient is different and so is their wish upon information needed/wanted prior to making important decisions.
Well designed and conducted clinical trials are essential to determine the effectiveness of a promising new drug or intervention. Many gynaecologic oncology departments have studies open for inclusion during the course of your cancer treatment. Talk to your doctor(s) about your thoughts regarding possible inclusion in a clinical trial now or in the future.
Author: Kristine Madsen, MD, Medical Director NSGO-CTU


