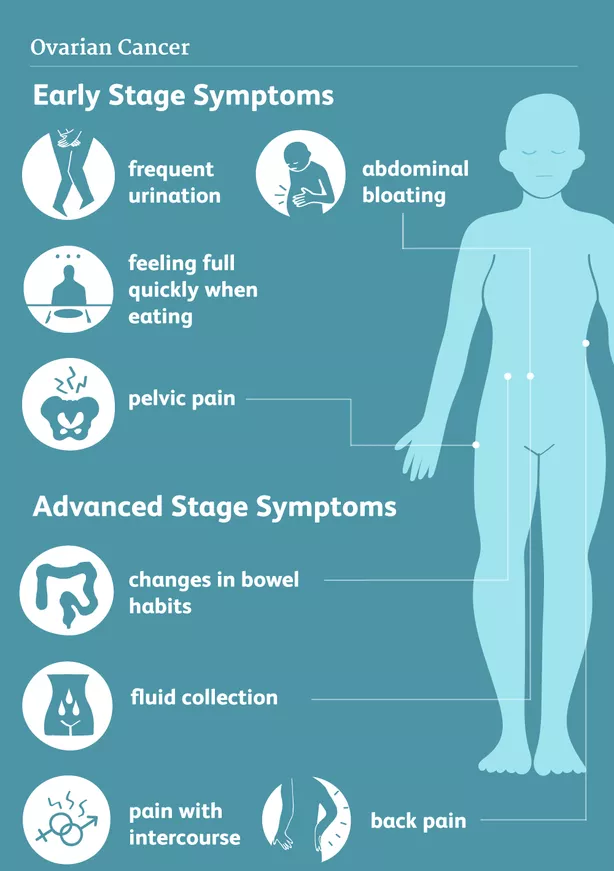
Ovarian cancer is predominantly diagnosed in stage 3 and 4. Symptoms are often unspecific and may include pelvic/abdominal symptoms like discomfort, pain, obstipation, bloating (due to fluid (ascites)), urinary tract irritation/infection, dyspnea, and deep venous thrombosis.
Patients presenting to their primary physician with symptoms indicating ovarian tumor, should be referred for specialized gynaecological evaluation with gynaecological examination, vaginal ultrasound and blood tests incl. the tumor marker Ca-125, which is used for ovarian cancer.
Verification of the diagnosis and histological subtype is required. The gynaecologist will retrieve tumor tissue sample(s) (biopsies) either by ultrasound-guided biopsy, or laparoscopy.
For final staging of ovarian cancer, an imaging examination must be performed to determine the extent of disease. The modality of choice varies between locations between PET/CT, CT, and MRI.
If larger quantities of fluids has accumulated in the abdomen (ascites) or chest (pleural effusion), patients can experience marked abdominal swelling, gastrointestinal irregularities or shortness of breath.
It can be indicated to drain the fluid from the abdomen or chest with a needle (paracentesis or thoracentesis) to alleviate severe symptoms. The extracted fluid may contain cancerous cells, and can be send for histological examination to document the diagnosis, if indicated.
Multi Disciplinary Team (MDT) Conference: When results from abovementioned examinations are available, best advised treatment strategy is discussed between doctors in the Multi Disciplinary Team. If initial surgery (Primary debulking Surgery (PDS)) is deemed technically and medically feasible, this will be recommended initially.
Patients with stage 4 ovarian cancer can start their cancer treatment with chemotherapy. The aim is to decrease the tumor bulk, before undergoing interval surgery later (Interval Debulking Surgery (IDS)).